
IVD Contract Manufacturing
We offer extensive contract development and manufacturing services. Our manufacturing facility is FDA (BLA) licensed and ISO 13485:2016 certified.
infor@avioq.com 1.919.314.5535

Specializing in the development, production and distribution of quality IVD products.
We provide flexible contract development and manufacturing services in an FDA licensed and ISO 13485 certified environment.
Apply our IVD product development experience and proven quality system to shorten your timeline. We have experience in all phases of IVD product development, starting from raw material development and finishing with an FDA approved and/or CE marked IVD.
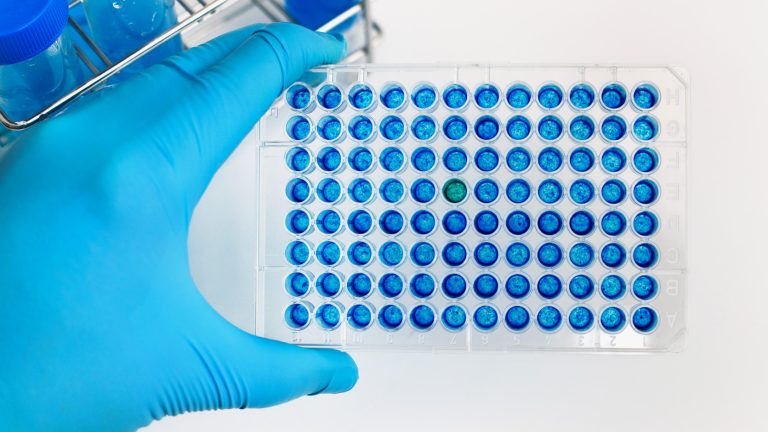

We offer extensive contract development and manufacturing services. Our manufacturing facility is FDA (BLA) licensed and ISO 13485:2016 certified.
Partner with a company that is flexible and able to move quickly to meet your assay development requirements and project timelines. Choose Avioq as your guide and partner for Companion Diagnostics Services to custom develop and commercialize your CDx project.
Bio-manufacturing and analytical capabilities include recombinant protein production either in mammalian or bacterial cells, monoclonal antibodies produced in hybridomas, and viral lysates/virus produced from large-scale cell cultures.
Browse our various recombinant antigens and inactivated viruses used for research or application in your diagnostic product. These products range from HIV-1 and HIV-2 antigens to HTLV-I and HTLV-II viral lysates.